Highlighted Publications
-
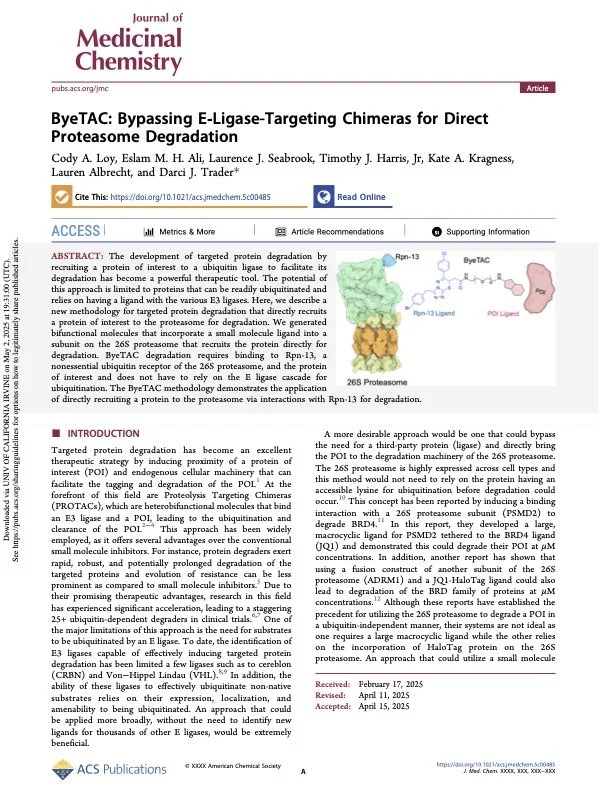
ByeTAC: Bypassing E-Ligase Targeting Chirmeras for Direct Proteasome Degradation
Here, we describe a new methodology for targeted protein degradation that directly recruits a protein of interest to the proteasome for degradation. ByeTAC degradation requires binding to Rpn-13, a nonessential ubiquitin receptor of the 26S proteasome, and the protein of interest and does not have to rely on the E ligase cascade for ubiquitination. The ByeTAC methodology demonstrates the application of directly recruiting a protein to the proteasome via interactions with Rpn-13 for degradation.
-

Immunoproteasome as a Target for Prodrug
Immunoproteasome (iCP) is a proteasome isoform that is expressed under inflammatory conditions such as cytokine interferon-γ exposure. The iCP has different catalytic subunits other than the standard CP (standard core particle), allowing the production of major histocompatibility complex class I (MHC-I) compatible peptides for eventual T-cell activation. This report highlights that the iCP is a viable prodrug target, and its activity can be used to release a variety of cargo in cells expressing the iCP.
-

Degradation of Abl Utilizing an Immunoproteasome N-Degron Prodrug
Targeted protein degradation is an emerging therapeutic strategy that leverages the cell’s natural protein clearance pathways to eliminate proteins of interest (POIs). One common approach involves bifunctional molecules that link an E3 ligase ligand to a POI ligand. However, these large molecules often suffer from poor cell permeability, and expanding ligands to new E3 ligases remains a challenge. As an alternative, we explored the use of N-degrons, single amino acid signals attached to small molecules, to recruit E3 ligases and induce POI ubiquitination and degradation. To enhance tissue selectivity and stability, we developed a caged N-degron degrader incorporating a short peptide sequence recognized by the immunoproteasome (iCP).
Full List of Publications
2025
19. Harris, T.J.*; Loy, C.A.*; Trader, D.J. CAPTaIN: Capped and Protected Targeted Immunoproteasome N-Degrons. 2025, Under Review, Proc. Natl. Acad. Sci. U.S.A. *Co-First Author
18. Loy, C.A.; Gu Y.; Benavente, C.A.; Trader, D.J. From Probe to Prodrug: Immunoproteasome Activity as a Trigger for Disease-Selective Therapeutics. 2025, Under Review, Sig Transduct Target Ther
17. Loy, C.A.; Trader, D.J. From Breakdown to Breakthrough: Immunoproteasomes Could Revolutionize Immunotherapies. J. Med. Chem. 2025, In Revision
16. King, H.D.; Loy, C.A.; Lyon, A.M.; Trader, D.J. A Prodrug for Immunoproteasome-Triggered Antigen Presentation. J. Med. Chem. 2025, Under Review
15. Loy, C.A.*; Harris Jr., T.J.*; Vinogradsky, S.E.; Trader, D.J. Degradation of Abl Utilizing an Immunoproteasome N-Degron Prodrug. J. Med. Chem. 2025, 68, 16, 17565–17573 *Co-First Author
14. Loy, C.A.; Harris Jr, T.J.; Trader, D.J. Synthesis and Characterization of ByeTACs (Bypassing E Ligase Targeting Chimeras) Current Protocols. 2025, (1) 5 (8), e70196 *Invited Protocol
13. Muli, C.M.; Loy, C.A.; Trader, D.J. Exploring the Immunoproteasome’s Substrate Preferences for Improved Hydrolysis and Selectivity. RSC Chem. Bio., 2025, In Revision
12. Loy, C.A., Ali, E.M.H; Seabrook, L.J.; Harris, T.J.; Kragness, K.A.; Albrecht, L.V.; Trader, D.J. ByeTAC: Bypassing E-Ligase Targeting Chimeras for Direct Proteasome Degradation. J. Med. Chem. 2025, 68, 9, 9694–9705
11. Muli, C.M.*; Loy, C.A.*; Trader, D.J. Immunoproteasome as a Target for Prodrugs. J. Med. Chem. 2025, 68, 6, 6507–6517 *Co-First Author
2024
10. Talele, S.; Gonzalez, S.; Trudeau, J.; Junaid, A.; Loy, C.A.; Altman, R.A.; SjÖgren, B. A Phenotypic High-Throughput Screen Identifies Small Molecule Modulators of Endogenous RGS10 in BV-2 Cells. J. Med. Chem. 2024, 67, 22, 20343–20352
9. Loy, C.A.; Trader, D. J. Synthesis and Application of a Caged Bioluminescent Probe for the Immunoproteasome. Current Protocols. 2024, 4.11.
8. Halder, S*.; Loy, C.A.*, Trader, D.J., Development of a Clickable ONX-0914 Covalent Probe for Immunoproteasome Activity Analysis. ChemBioChem. 2024, e202400571. *Co-First Author.
7. Seabrook, L.J.; Franco, C.N.; Loy, C.A.; Fredlender, C.; Zimak, J.; Campos, M.; Nguyen, S.T.; Watson, L.R.; Levine, S.; Osman, J.; Khalil, M.F.; Sumigray, K.; Trader, D.J.; Albrecht, L.V. A Methyl Arginine Targeting Chimera for Lysosomal Degradation of Intracellular Proteins. Nat. Chem. Biol. 2024, 1-11 Cover – Nat. Chem. Bio. December 2024
6. Loy, C.A.; Trader, D. J. Caged Aminoluciferin Probe for Bioluminescent Immunoproteasome Activity Analysis. RSC Chemical Biology, 2024, 5, 877-883
5.Loy, C.A.; Trader, D.J. Primed for Interactions: Investigating the Primed Substrate Channel of the Proteasome for Improved Molecular Engagement. Molecules, 2024, 29, 3356.
2023
4. Loy, C.A.; Muli, C.M.; Ali, E.M.H.; Xie, D.; Ahmed, M.H.; Post, C.B.; Trader, D.J. Discovery of a non- covalent ligand for Rpn-13, a therapeutic target for hematological cancers, Bioorg. Med. Chem. Lett., 2023, 95, 129485.
2021
3. Loy, C.; Holthoff, J. M.; Weiss, R.; Huber, S. M.; Rosokha, S. V. “Anti-Electrostatic” Halogen Bonding in Solution. Chem. Sci. 2021, 12 (23), 8246–8251.
2. Miller, D. K.; Loy, C.; Rosokha, S. V. Examining a Transition from Supramolecular Halogen Bonding to Covalent Bonds: Topological Analysis of Electron Densities and Energies in the Complexes of Bromosubstituted Electrophiles. ACS Omega. 2021, 6 (36), 23588–23597.
2020
1. Loy, C.; Zeller, M.; Rosokha, S.V. Halogen Bonding in the Complexes of Brominated Electrophiles with Chloride Anion: From a Weak Supramolecular Interaction to a Covalent Br-Cl Bond. Crystals. 2020, 10.